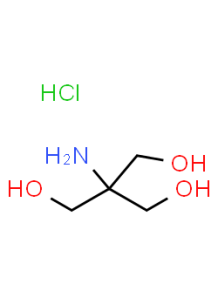
TRIS HCl (Tris Hydrochloride)
also known as Tris Hydrochloride, is a chemical compound commonly used in various scientific and industrial applications

also known as Tris Hydrochloride, is a chemical compound commonly used in various scientific and industrial applications
TRIS HCl, also known as Tris Hydrochloride, is a chemical compound commonly used in various scientific and industrial applications. Here are some key details about TRIS HCl:
1. Chemical Structure and Properties:
- TRIS HCl is the hydrochloride salt of tris(hydroxymethyl)aminomethane (TRIS or Tris).
- It is a white, crystalline powder that is highly soluble in water and other polar solvents.
- TRIS HCl has a pH range of around 7-9 when dissolved in water, making it useful as a buffer solution.
2. Applications:
- Buffer Solutions: TRIS HCl is widely used as a component in buffer solutions, particularly in biochemistry and molecular biology applications, such as in the preparation of reagents for gel electrophoresis.
- Protein Purification: TRIS HCl is used in the purification of proteins, as it can help maintain the proper pH and ionic environment during various chromatographic techniques.
- Cell Culture: TRIS HCl is sometimes included in cell culture media to help maintain a suitable pH for cell growth and maintenance.
- Analytical Techniques: TRIS HCl is used in various analytical methods, such as spectrophotometry and enzyme activity assays, where a controlled pH environment is required.
3. Advantages:
- Buffering Capacity: TRIS HCl provides a good buffering capacity over a wide pH range, making it useful for maintaining a stable pH in various applications.
- Biocompatibility: TRIS HCl is generally considered a biocompatible compound and is commonly used in biological and medical applications.
- Versatility: TRIS HCl can be used in a variety of laboratory and industrial settings, from biochemistry and cell biology to materials science and water treatment.
4. Considerations:
- pH Dependence: The pH of TRIS HCl solutions can be affected by factors such as temperature and the presence of other ions, so the pH should be carefully monitored and adjusted as needed.
- Potential Interactions: TRIS HCl may interact with certain compounds or materials, so its compatibility should be evaluated when used in specific applications.
Overall, TRIS HCl is a versatile and widely used buffer component in various scientific and industrial applications, particularly in the fields of biochemistry, molecular biology, and analytical chemistry.
| Mechanism | - |
| Appearance | - |
| Longevity | - |
| Strength | - |
| Storage | - |
| Shelf Life | - |
| Allergen(s) | - |
| Dosage (Range) | - |
| Recommended Dosage | - |
| Dosage (Per Day) | - |
| Recommended Dosage (Per Day) | - |
| Mix Method | - |
| Heat Resistance | - |
| Stable in pH range | - |
| Solubility | - |
| Product Types | - |
| INCI | - |
Purchase History for
Cart
No products