Total Mold and Yeast Counting Service
- Product Code: 127390
Total Mold and Yeast Counting Service
description Service Overview
Total Mold and Yeast Counting Procedure
A. Materials and Reagents
- Sample: The food, environmental, or other material to be analyzed.
- Diluent: Sterile diluent such as 0.1% peptone water, phosphate-buffered saline (PBS), or another suitable medium.
- Dilution Blanks: Sterile tubes or bottles for making serial dilutions.
- Pipettes and Pipette Tips: Sterile, calibrated pipettes for accurate volume transfers.
- Homogenizer or Stomacher: For thoroughly mixing solid samples.
- Agar Medium:
- Sabouraud Dextrose Agar (SDA) is commonly used.
- Alternatively, DG18 agar may be used especially when working with samples that have low water activity.
- Antibiotics (Optional): Such as chloramphenicol or gentamicin added to the medium to inhibit bacterial growth.
- Sterile Petri Dishes
- Incubator: Set at the appropriate temperature (typically 25–30°C).
- Colony Counter: Manual or automated.
B. Step-by-Step Procedure
1. Sample Preparation
- For Solid Samples:
- Weigh an appropriate amount (commonly 10 g) of the sample into a sterile blender bag or stomacher bag.
- Add 90 mL of sterile diluent to achieve a 1:10 initial dilution.
- Homogenize the sample thoroughly using a stomacher or equivalent device.
- For Liquid Samples:
- Mix the sample well.
- If necessary, dilute an aliquot in sterile diluent (e.g., 1:10) to reduce microbial load.
2. Serial Dilutions
- Prepare a series of tenfold dilutions by transferring 1 mL of the homogenized sample into 9 mL of sterile diluent.
- Mix each dilution well (vortex or shake) to ensure even distribution of microorganisms.
- Continue this process until you reach dilutions that are expected to yield countable colonies (generally plates with 30–300 colonies).
3. Plate Inoculation
You can choose one of the two common plating methods:
A. Pour Plate Method:
- Dispense Sample:
- Pipette 1 mL of the appropriate dilution into a sterile Petri dish.
- Add Agar:
- Pour 15–20 mL of molten, cooled (approximately 45–50°C) agar medium (e.g., SDA with antibiotics) into the dish.
- Mix:
- Gently swirl the dish to mix the sample with the agar.
- Allow to Solidify:
- Let the agar solidify at room temperature.
B. Spread Plate Method (if preferred):
- Plate Preparation:
- Pour the agar medium into plates and allow it to solidify.
- Dispense Sample:
- Pipette an appropriate volume (commonly 0.1 or 1 mL) of the dilution onto the agar surface.
- Spread Sample:
- Use a sterile spreader (e.g., glass or plastic spreader) to evenly distribute the sample over the plate surface.
Tip: The pour plate method is often preferred for mold and yeast counts because it allows colonies to grow both on and within the agar, which can enhance recovery.
4. Incubation
- Incubate the inoculated plates at 25–30°C.
- Incubation times typically range from 5 to 7 days.
- Molds often require the full 7 days to develop visible colonies.
- Yeasts may grow faster; however, when counting both groups together, use the longer incubation time.
- Monitor the plates periodically for colony development.
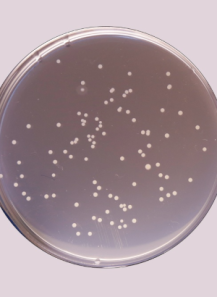
5. Colony Counting
- After incubation, select plates that have between 30 and 300 colonies for accurate enumeration.
- Count the colonies manually or use a colony counter.
- If the colonies are too numerous to count on a particular dilution, use counts from a higher dilution.
timeline Service Steps
| Step | Procedure | Expected Result |
|---|---|---|
| info Service steps will be provided upon request | ||
Cart
No products
Subtotal:
0.00
Total
0.00
THB