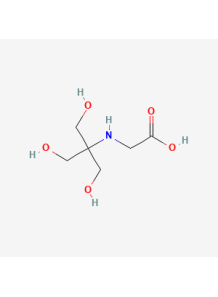
Tricine
also known as N-[Tris(hydroxymethyl)methyl]glycine, is a chemical compound that is commonly used as a buffer in biochemical and molecular biology applications
Cart
No products
No products
also known as N-[Tris(hydroxymethyl)methyl]glycine, is a chemical compound that is commonly used as a buffer in biochemical and molecular biology applications
Tricine, also known as N-[Tris(hydroxymethyl)methyl]glycine, is a chemical compound that is commonly used as a buffer in biochemical and molecular biology applications. Here are some key details about tricine:
1. Chemical Structure and Properties:
- Tricine is a zwitterionic buffer compound, meaning it contains both positively and negatively charged groups within the same molecule.
- It is a white, crystalline powder that is highly soluble in water and other polar solvents.
- Tricine has a pKa value of around 8.1, making it useful for maintaining a slightly alkaline pH range.
2. Applications:
- Protein Electrophoresis: Tricine is commonly used as a buffer component in SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) for the separation and analysis of proteins, particularly small proteins and peptides.
- Enzyme Assays: Tricine buffers are used in various enzyme activity assays, as they can help maintain the optimal pH environment for enzymatic reactions.
- Cell Culture: Tricine may be included in cell culture media to help maintain the desired pH range for cell growth and metabolism.
- Biochemical Reactions: Tricine can be used as a buffer in a wide range of biochemical reactions, such as DNA/RNA manipulation, protein purification, and enzyme kinetics studies.
3. Advantages:
- pH Buffering Range: Tricine is effective in maintaining a pH range of approximately 7.4-9.0, making it suitable for applications that require a slightly alkaline environment.
- Compatibility: Tricine is generally considered a biocompatible buffer and is widely used in biological and biochemical applications.
- Solubility: Tricine has good solubility in water and other polar solvents, allowing for easy preparation of buffer solutions.
4. Considerations:
- Temperature Dependence: The pH of tricine buffer solutions can be affected by temperature, so the pH should be carefully monitored and adjusted as needed.
- Potential Interactions: Like any buffer, tricine may interact with certain compounds or materials, so its compatibility should be evaluated for specific applications.
Overall, tricine is a versatile and commonly used buffer in various biochemical and molecular biology applications, particularly in the areas of protein analysis, enzyme assays, and cell culture.
| Mechanism | - |
| Appearance | - |
| Longevity | - |
| Strength | - |
| Storage | - |
| Shelf Life | - |
| Allergen(s) | - |
| Dosage (Range) | - |
| Recommended Dosage | - |
| Dosage (Per Day) | - |
| Recommended Dosage (Per Day) | - |
| Mix Method | - |
| Heat Resistance | - |
| Stable in pH range | - |
| Solubility | - |
| Product Types | - |
| INCI | - |