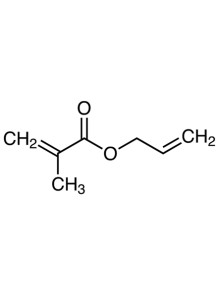
a chemical compound with the formula C7H10O2. It is an ester formed from methacrylic acid and allyl alcohol. The compound is characterized by the presence of both a methacrylate group and an allyl group, which makes it a versatile monomer in polymer chemistry
Allyl methacrylate is a chemical compound with the formula C7H10O2. It is an ester formed from methacrylic acid and allyl alcohol. The compound is characterized by the presence of both a methacrylate group and an allyl group, which makes it a versatile monomer in polymer chemistry.
Structure:
Properties:
-
Molecular Weight: 126.15 g/mol
-
Appearance: Typically a clear, colorless liquid
-
Boiling Point: Approximately 150-152°C (302-306°F)
-
Density: Around 0.94 g/cm³
Uses:
-
Polymerization: Allyl methacrylate is commonly used as a crosslinking agent in the production of polymers and copolymers. The presence of both methacrylate and allyl groups allows it to participate in free radical polymerization, leading to the formation of crosslinked networks.
-
Adhesives and Coatings: It is used in the formulation of adhesives, coatings, and sealants due to its ability to form strong, durable bonds.
-
Dental Materials: It is also used in dental composites and restorative materials.
-
Optical Materials: The compound is used in the production of optical lenses and other materials requiring high transparency and durability.
Safety:
-
Handling: Allyl methacrylate should be handled with care, as it can be irritating to the skin, eyes, and respiratory system. Proper personal protective equipment (PPE) such as gloves, goggles, and lab coats should be used.
-
Storage: It should be stored in a cool, dry place, away from sources of ignition and incompatible materials such as strong oxidizing agents.
Polymerization:
When polymerized, allyl methacrylate can form highly crosslinked polymers, which are often used in applications requiring high strength and chemical resistance. The allyl group can also participate in additional reactions, providing further functionalization opportunities.
Overall, allyl methacrylate is a valuable monomer in the production of various polymeric materials, contributing to the development of products with enhanced mechanical and chemical properties.