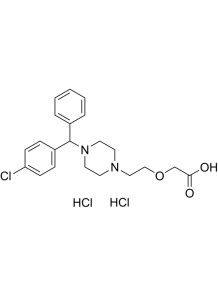
Cetirizine dihydrochloride
recently found to have good efficacy in reducing hair loss
Cart
No products
No products
recently found to have good efficacy in reducing hair loss
this item Can be sold only to hospitals, clinics, drug factories, pharmacists, doctors for use in research only. The purchaser must submit the following documents: Natural Person: Medical certificate or pharmacist Juristic Person: Documents received around the drug manufacturing site or a document certifying the hospital (clinic/hospital)
Cetirizine dihydrochloride recently found to have good efficacy in reducing hair loss
Pharmaceutical sciences branch, Islamic Azad University, Tehran, Iran;2Center for Research & Training in Skin Diseases & Leprosy, Tehran University ofMedical Sciences, Tehran, Iran.; 3Department of Pharmaceutics, Faculty of Pharmacy,Tehran University of Medical Sciences, Tehran, Iran;4Legacy Healthcare SA, Epalinges, Switzerland
Study Purpose: Cetirizine, an antihistamine, is hypothesized to benefit hair growth by inhibiting prostaglandin D2 (which promotes hair loss) and stimulating prostaglandin E2 (which promotes hair growth). The study aimed to evaluate the effectiveness and safety of cetirizine for AGA compared to minoxidil, a well-established hair growth treatment.
Methodology:
Results:
Conclusion: Cetirizine 1% is effective for AGA treatment and may offer a safer alternative for those who experience side effects from minoxidil. Further research with larger samples and varied concentrations could provide more insights.
Use: use in medicine only Not allowed to use in cosmetics
Product characteristics: White powder, soluble in water.
Storage: For long term storage keep in refrigerator Do not expose to sunlight, heat, seal the lid tightly, shelf life at least 2 years.
Chemical Name : Cetirizine dihydrochloride
| Mechanism | - |
| Appearance | - |
| Longevity | - |
| Strength | - |
| Storage | - |
| Shelf Life | - |
| Allergen(s) | - |
| Dosage (Range) | - |
| Recommended Dosage | - |
| Dosage (Per Day) | - |
| Recommended Dosage (Per Day) | - |
| Mix Method | - |
| Heat Resistance | - |
| Stable in pH range | - |
| Solubility | - |
| Product Types | - |
| INCI | - |