Promethazine HCI
Strong H₁-histamine receptor antagonist; moderate muscarinic (anticholinergic) blockade; weak antagonism at 5-HT₂, D₂ and α-adrenergic receptors; non-competitive NMDA receptor antagonist
Cart
No products
No products
Strong H₁-histamine receptor antagonist; moderate muscarinic (anticholinergic) blockade; weak antagonism at 5-HT₂, D₂ and α-adrenergic receptors; non-competitive NMDA receptor antagonist
For Research Only
A. Chemical & Physical Properties
A1. Identity & Structure
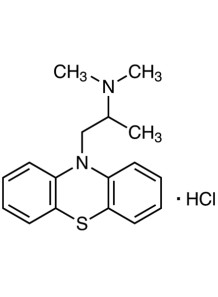
Empirical formula: C₁₇H₂₀N₂S·HCl
IUPAC name: (RS)-N,N-Dimethyl-1-(10H-phenothiazin-10-yl)propan-2-amine monohydrochloride
A2. Molecular Weight & CAS
CAS No.: 58-33-3
M.W.: 320.88 g/mol
A3. pKa
pKa (conjugate acid): 9.1
A4. Appearance & Stability
White to faint-yellow crystalline powder; odorless; slow oxidation in air (blue discoloration)
A5. Solubility & pH
Freely soluble in water (≥100 mg/mL at 25 °C) and soluble in alcohol
Typical oral solution pH: 4.7–5.2
B. Pharmacology & Uses
B1. Mechanism of Action
Strong H₁-histamine receptor antagonist; moderate muscarinic (anticholinergic) blockade; weak antagonism at 5-HT₂, D₂ and α-adrenergic receptors; non-competitive NMDA receptor antagonist
B2. Clinical Indications
Allergic reactions (hay fever, urticaria)
Nausea/vomiting (motion sickness, postoperative, chemotherapy)
Sedation/insomnia
Adjunct in common cold, hemolytic disease of the newborn
Anxiety before surgery
B3. Routes & Dosage Forms
Oral tablets: 12.5–25 mg
Oral syrup: typically 6.25 mg/5 mL
Rectal suppositories: 12.5 mg
Injectable solution: 25 mg/mL
C. Pharmacokinetics
C1. Absorption
~88% absorbed; first-pass reduces absolute bioavailability to ~25%
C2. Distribution
~93% plasma protein bound
C3. Metabolism & Elimination
Hepatic glucuronidation and sulfoxidation; t½ 10–19 h; excreted via urine and bile
D. Formulation Considerations
D1. pH & Buffering
Maintain formulation pH 4.7–5.2 (e.g., citrate buffer) to ensure salt stability and solubility
D2. Light & Oxidation
Protect from light/air; use amber glass or opaque containers to minimize blue oxidation
E. Safety & Side Effects
E1. Common AEs
Sedation/drowsiness, dizziness, dry mouth, blurred vision, urinary retention
E2. Warnings & Contraindications
Contraindicated in children < 2 years (respiratory depression), hypersensitivity, narrow-angle glaucoma, prostatic hypertrophy
Caution in elderly (anticholinergic burden), hepatic/renal impairment
F. Regulatory Status
Prescription-only in the US; POM in the UK; OTC in Canada; S3 in Australia
| Mechanism | - |
| Appearance | - |
| Longevity | - |
| Strength | - |
| Storage | - |
| Shelf Life | - |
| Allergen(s) | - |
| Dosage (Range) | - |
| Recommended Dosage | - |
| Dosage (Per Day) | - |
| Recommended Dosage (Per Day) | - |
| Mix Method | - |
| Heat Resistance | - |
| Stable in pH range | - |
| Solubility | - |
| Product Types | - |
| INCI | - |